What Keeps Electrons in Orbit Around the Nucleus
Second - at least in the molecular orbital model - the stability of the chemical bond is a purely quantum effect created by electron exchange which has no classical analog. Both have opposite charges.
What Force Keeps Electrons Moving Around The Nucleus Of An Atom Quora
Opposites attract and so the atom holds together.

. In addition to keeping drawings stuck to the refrigerator the electromagnetic force also keeps electrons in orbit. Since the nucleus is positively charge the electrons which. There exists an electrostatic force between the positive nucleus and negative electrons.
In short electrical attraction to the nucleus is what keeps the electron in orbit and this attraction doesnt cost energy. At the beginning of the 1900s it became apparent that the best way to model an atom was to conceive of a very dense central part the nucleus with a positive charge and almost all the mass surrounded at a relatively large distance compared to the size of the nucleus by a cloud of elementary negatively-charged electrons. One important point to note is that electrons dont orbit the nucleus of an atom they occupy orbitals.
This electrostatic force supplies the necessary centripetal force for. The electrons do not orbit the nucleus in the manner of a planet orbiting the sun but instead exist as standing waves. All it needs is the electrostatic attraction between the positively-charged protons in the atoms nucleus and the negatively-charged electron.
In short electrical attraction to the nucleus is what keeps the electron in orbit and this attraction doesnt cost energy. These very light electrons would be in orbit around. The charge of the nucleus is positive and the charge of the electron is negative.
Electrons are kept in the orbit around the nucleus by the electromagnetic force because the nucleus in the center of the atom is positively charged. The proposal first made in 1913 that the centrifugal force of the revolving electron just exactly balances the attractive force of the nucleus in analogy with the centrifugal force. All it needs is the electrostatic attraction between the positively-charged protons in the atoms nucleus and the negatively-charged electron.
Classical physicists wondered that the electron didnt run out of energy. Electrons have a negative charge and the nucleus has a positive charge. Hence They attract so if the electron moves sideways at the right speed it will bend toward the nucleus and just keep on bending in a circle the same as a satellite orbiting earth.
First being quantum particles electrons do not orbit nuclei in the planetary sense. Electrostatic attractions keep electrons in their orbit around the nucleus of an atom. A bond more or less is a region of electron density.
The nuclear force of attraction between atomic nuclei and electron is responsible to keep the electron in orbit. In addition to keeping drawings stuck to the refrigerator the electromagnetic force also keeps electrons in orbit. Since the nucleus is positively charge the electrons which.
Electrostatic attractions keep electrons in their orbit around the nucleus of an atom. Electrons have a negative charge and the nucleus has a positive charge. The picture of electrons orbiting the nucleus like planets around the sun remains an enduring one not only in popular images of the atom but also in the minds of many of us who know better.
Like gravity acting on planets an electromagnetic force attracts the orbiting electron to the nucleus. But the reason that electron keeps moving in circular path around nucleus is due to centripetal force. Opposites attract and so the atom holds together.
The electrons are never in a single point location although the probability of interacting with the electron at a single point can be found from the wave function of the electron. The short answer is that its the electromagnetic interaction or force.

Atom Orbits And Energy Levels Britannica
What Provides Energy To Electrons To Orbit The Nucleus Quora
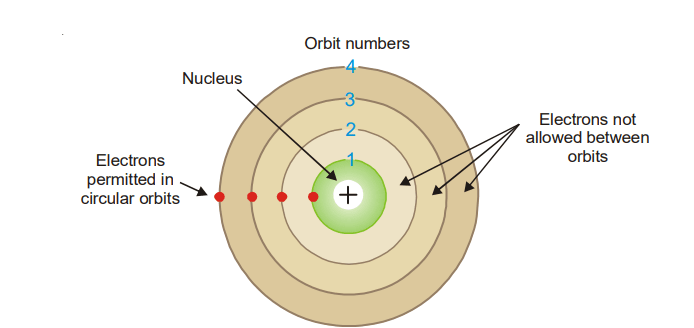

Comments
Post a Comment